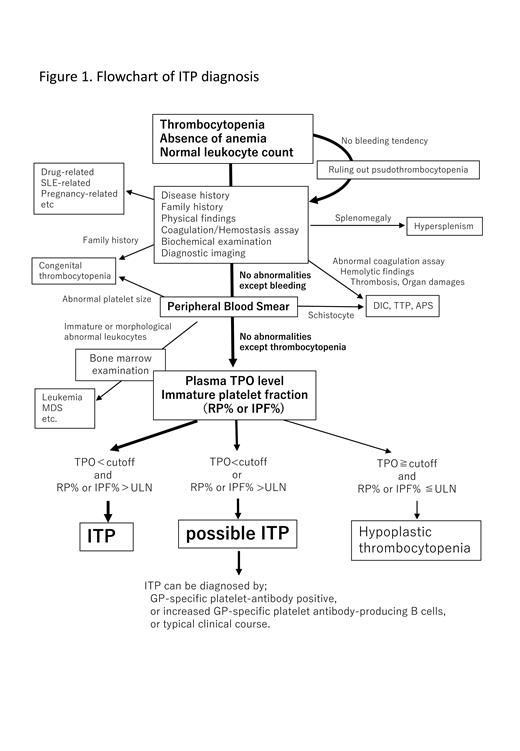
Primary immune thrombocytopenia (ITP) is an autoimmune disorder characterized by isolated thrombocytopenia due to accelerated platelet destruction and impaired platelet production. Diagnosis of ITP is still challenging because ITP has been diagnosed by exclusion. Exclusion of thrombocytopenia due to bone marrow failure is especially important in Japan because of high prevalence of aplastic anemia compared to Western countries. Hence, we propose new diagnostic criteria involving the measurement of plasma thrombopoietin (TPO) levels and percentage of immature platelet fraction (RP% or IPF%) as shown in Fig. 1. These biomarkers reflect the pathology of ITP, in which shortened platelet lifespan associated with increased platelet destruction and relatively maintained platelet production.
Diagnostic criteria is as follows:
1. ALL of the following *1
・Thrombocytopenia (<100,000/μL)
・Absence of anemia (excluding anemia due to bleeding and/or iron deficiency)
・Normal leukocyte count (but may present with mild abnormal leukocyte count)
・No morphological evidence of dysplasia in any blood cell type in a blood smear
2. Normal or slightly increased plasma TPO level (<cutoff)
3. Elevated percentage of immature platelet fraction (RP% or IPF%; > upper limit of normal)
4. Absence of other conditions that potentially cause thrombocytopenia including secondary ITP ⋆2
● A diagnosis of ITP is made if conditions 1 - 4 above are all met (ITP)
● Cases in which criterion 2 or 3 is not met or unavailable are defined as “possible ITP, pITP”. A diagnosis of ITP can be made if the patient is a glycoprotein (GP)-specific platelet-antibody positive, increased GP-specific platelet antibody-producing B cells, or typical clinical course.
(Footnotes)
*1 Bone marrow examination is strongly recommended to rule out other diseases if peripheral blood demonstrates any of the following findings: leukocyte count <3,000 or ≥10,000/μL, MCV ≥110, neutrophils <30% or lymphocytes ≥50%, presence of immature leukocytes
*2 Includes drug-related thrombocytopenia, radiation injury, aplastic anemia, myelodysplastic syndrome (MDS), paroxysmal nocturnal hemoglobinuria (PNH), systemic lupus erythematosus (SLE), leukemia, malignant lymphoma, bone marrow metastasis of cancer cells, disseminated intravascular coagulation (DIC) , thrombotic thrombocytopenic purpura (TTP), hypersplenism, megaloblastic anemia, sepsis, infections (tuberculosis, etc.), sarcoidosis, and huge hemangioma such as Kasabach-Merritt syndrome. Congenital diseases involving thrombocytopenia include Bernard-Soulier syndrome, Wiskott-Aldrich syndrome, MYH9-related disease and Upshaw-Schulman syndrome.
Sensitivity and specificity analysis of this criteria was performed by using data of previously performed prospective study (Kuwana et al. J Thromb Haemost 2006). In this study plasma TPO level were measured by R&D Systems ELISA Kit and RP% were measured by flow cytometry. 112 thrombocytopenic patients who were initially suspected ITP (final diagnosis: ITP 88, aplastic anemia 11, MDS 10, others 3) were included in the study. The sensitivity and specificity of “ITP” who met all 4 conditions of the new criteria were 53.4% (47/88) and 95.8% (23/24), respectively. If cases of diagnosed as “ITP” or “pITP” by the new criteria were considered as ITP, sensitivity and specificity were 94.3% (83/88) and 75.0% (18/24), respectively. More importantly, these criteria exclude aplastic anemia completely from true ITP. For clinical diagnostic use, new CLEIA-based TPO measurement kit (MBL, Japan) has recently been developed in Japan. However, it is still crucial to carefully differentiate ITP from other thrombocytopenic diseases before applying these biomarkers. These new criteria enable us to clearly differentiate ITP from aplastic anemia and other forms of hypoplastic thrombocytopenia, and can be highly useful in clinical practice for avoiding unnecessary bone marrow examination as well as for appropriate selection of treatments.
Disclosures
Kashiwagi:Sysmex: Honoraria, Research Funding; Novartis: Honoraria; Kissei Phamaceutical: Honoraria; Argenix Japan: Honoraria. Yamanouchi:Chugai pharmaceutical: Honoraria. Hato:Novartis: Honoraria. Tomiyama:Sysmex: Consultancy; Novartis: Honoraria; Kyowa Kirin: Honoraria; Kissei Phamaceutical: Honoraria.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal